BioVAT-HF-DZHK20
Study information
BioVAT-HF-DZHK20
- Recruiting status
-
Recruiting ongoing
- Recruitment start
02/2021
- Patients
53
- Clinical Trials Registration
- Category
Early clinical study
- DZHK Funding
EUR 1.185.447
- Links
Operative contact
Main study center
Florian Walker, MD
biovat.info@med.uni-goettingen.de
Safety and Efficacy of Induced Pluripotent Stem Cell-derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-DZHK20)
The BioVAT-HF-DZHK20 trial is investigating under which conditions engineered human myocardium is safe to use in the treatment of patients with end-stage heart failure, a condition known as terminal heart failure (NYHA III or IV). To do so, engineered human myocardium will be implanted onto the heart muscle by means of a minimally invasive thoracotomy, a surgical opening of the thorax through an incision between two ribs, or open-heart surgery. With this study, the scientists hope to gain new insights into whether this intervention leads to better heart function, what dosage is the correct one and where the implantation of the artificial heart tissue is most effective.

Publications
Engineered heart muscle allografts for heart repair in primates and humans. Jebran, AF., Seidler, T., Tiburcy, M. et al. Nature. 2025 Jan 29. doi: 10.1038/s41586-024-08463-0.
Principal Investigators
Principal investigator: Wolfram-Hubertus Zimmermann (Göttingen)
Press releases and news
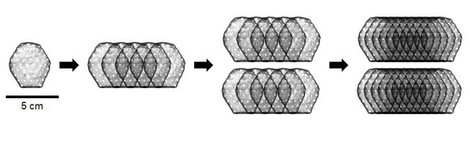
Heart patch in clinical trial: Dose finding completed (BioVAT-DZHK20 study)
The world's only clinical trial to implant artificial heart tissue in patients with heart failure to...
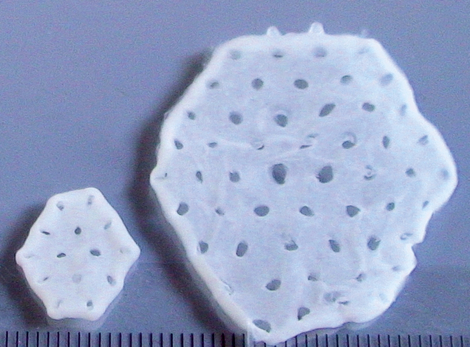
Start of First Clinical Trial on Tissue Engineered Heart Repair (Study BioVAT-DZHK20)
For the first time, engineered heart muscle (EHM) from human induced pluripotent stem cells (iPSCs)...
Study recruitment is available in these cities
The map only displays recruitment locations within Germany.
