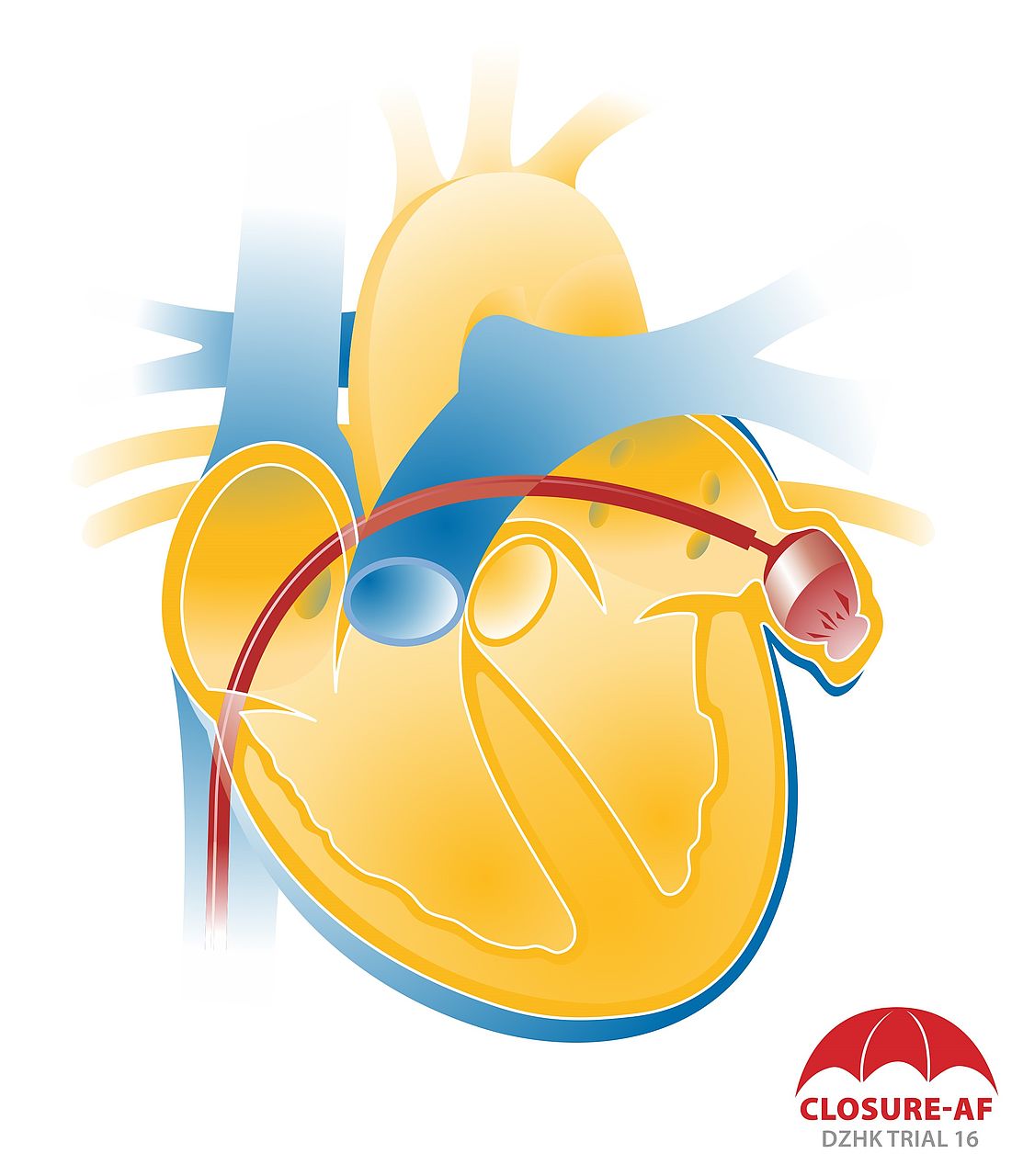
Atrial fibrillation is the most common cardiac arrhythmia. It particularly affects the elderly and is the most common cause of a stroke, because if the atria quiver, there is a risk of blood clots forming. More than 90 percent of cardiac blood clots are detected in the left atrial appendage, a sac in the left atrium of the human heart. From there, the clots can find their way into the blood vessels of the brain and cause a stroke. Patients with atrial fibrillation therefore receive anticoagulants that reduce the risk of a stroke by 70 percent. These are either vitamin K antagonists or non-VKA novel oral anticoagulants (NOACs). Both drug groups, however, are associated with an increased risk of severe bleeding. 20 to 25 percent of patients with atrial fibrillation can thus not persistently take NOACs.
Shielding from the risk of stroke
As an alternative to anticoagulant therapy, a procedure was thus developed in which the left atrial appendage is closed with a sort of mini parachute so that blood clots can no longer reach the brain or body from there. The closure is positioned using a catheter. Following the procedure, patients only have to take short-term antithrombotics until the closure heals.
Current scientific data from two smaller studies show that strokes can be successfully prevented using a left atrial appendage closure (LAAC). However, substantiated data on the benefit of this method for high-risk patients at high risk of bleeding and high risk of stroke are missing. “This procedure is an important alternative especially for this patient group”, explains principal investigator Professor Ulf Landmesser of Charité – Universitätsmedizin Berlin. For some of these patients, the risk of bleeding is so high that they can no longer take any anticoagulants at all. For this reason, the DZHK study CLOSURE-AF compares the benefit of the left atrial appendage closure with each best medical therapy for this high-risk group.
CLOSURE-AF is the largest global study on this issue: More than 1,500 patients are expected to be included in the study, and 17 DZHK sites and 45 further sites in Germany are participating in it. The Atrial Fibrillation Network e. V. is closely cooperating and assuming the regulatory project management of the study.
Starting from the first quarter of 2018, patients will be recruited for the study over a period of three years; in total, the study is expected to last five years. The results of the CLOSURE-AF study will be incorporated into the guidelines on the treatment of patients with atrial fibrillation and at high risk of stroke and bleeding.
Study: Left atrial appendage closure in patients with atrial fibrillation at high risk of stroke and bleeding compared to medical therapy: a prospective randomized clinical trial CLOSURE-AF
Principal investigator: Professor Dr. med. Ulf Landmesser, Charité – Universitätsmedizin Berlin, Director of the Medical Department of Cardiology, Campus Benjamin Franklin, Medical Directorate CC11 Cardiovascular Medicine
Project coordinator: Dr. med. Johannes Jakob Hartung, Charité – Universitätsmedizin Berlin, Medical Department of Cardiology, Campus Benjamin Franklin, Clinical Study Centre, closure-af(at)dzhk.de
Contact: Christine Vollgraf, Public Relations Officer, German Centre for Cardiovascular Research (DZHK), phone: +49 30 3456 529 02, presse(at)dzhk.de
You can find further information on the CLOSURE-AF study as well as a list of all participating study sites and contact partners at: https://closure-af.dzhk.de/