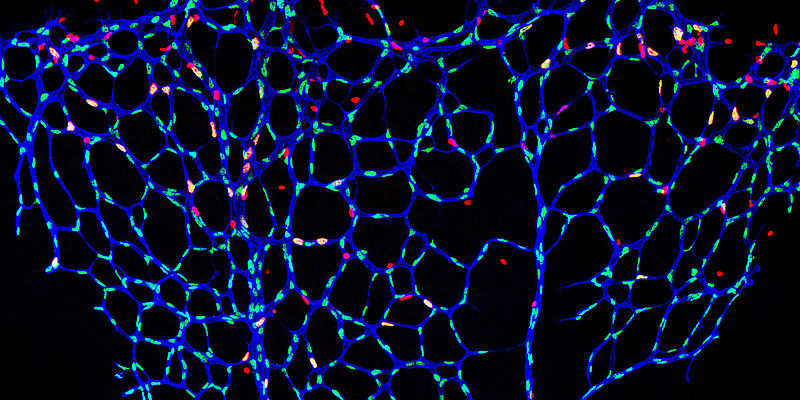
Diseases of the cardiovascular system are often associated with impaired function of the inner lining of blood vessels (endothelial dysfunction). The research group of Univ.-Prof. Dr. Philip Wenzel, Deputy Director of Cardiology I in the Center for Cardiology at the University Medical Center Mainz and Principal Investigator at the DZHK, has discovered that the protein tubulin-folding cofactor E (TBCE) plays a crucial role in this process. TBCE is one of the proteins that are important in giving cells structure and shape, allowing them to function normally.
Active substance can prevent stress reactions in the vessel
The researchers were able to show that a deficiency or mutation of the protein TBCE leads to a stress reaction in the vessel wall. The endoplasmic reticulum, the inner network of the cells, is affected. The vascular stress is associated with inflammation and increased vascular stiffness, among other things.
It was known from previous studies that the active substance TUDCA (tauroursodeoxycholic acid) can suppress stress reactions in the endoplasmic reticulum. In the Mainz study, targeted pharmacological therapy with TUDCA improved endothelial function in animal models, even when the protein TBCE was defective.
"The currently published work demonstrates the potential to gain important insights at the biotechnology location of Mainz with strategic use of research funds, which can have an immediate benefit for human healthcare," emphasizes Univ.-Prof. Dr. Thomas Münzel, Director of Cardiology I in the Center for Cardiology at Mainz University Medical Center.
Endothelial disorders are breeding grounds for atherosclerosis
The endothelium is the inner skin of the blood vessels and, like a fine membrane of specialized cells, covers the vascular bed throughout the body from the inside. It ensures the widening and narrowing of the vessels and thus a regulated blood flow in the body. Disorders of the endothelium are thus the breeding ground for atherosclerosis, coronary heart disease, heart attacks and strokes, as well as thromboses and pulmonary embolisms. Additional factors such as smoking and hypertension can exacerbate endothelial dysfunction.
The first clues to the now newly discovered mechanism regulating blood vessel function came from the analysis of genetic data from the Gutenberg Health Study (GHS). The study, conducted by the University Medical Center Mainz, has been collecting data on risk factors of cardiovascular diseases in the population of the Rhine-Main region for about 14 years, together with molecular biological measurements. "It is fascinating to see that the GHS is able to provide this information," reports Univ.-Prof. Dr. Philip Wenzel.
Results may be the basis for new therapy of endothelial dysfunction
"Furthermore, our experimental know-how made it possible to extract a previously unknown mode of function from the complex data. Our research results on TBCE provide promising indications that this mechanism can be influenced by drugs to improve vascular function in affected patients. This is particularly important because the current therapy of endothelial dysfunction with vitamins or trace elements has shown only insufficient success," explains Professor Wenzel.
Original publication: Efentakis et.al Tubulin-folding cofactor E deficiency promotes vascular dysfunction by increased endoplasmic reticulum stress. Eur Heart J. 2021 Jun 16:ehab222.
Source: Pressemitteilung Universitätsmedizin Mainz